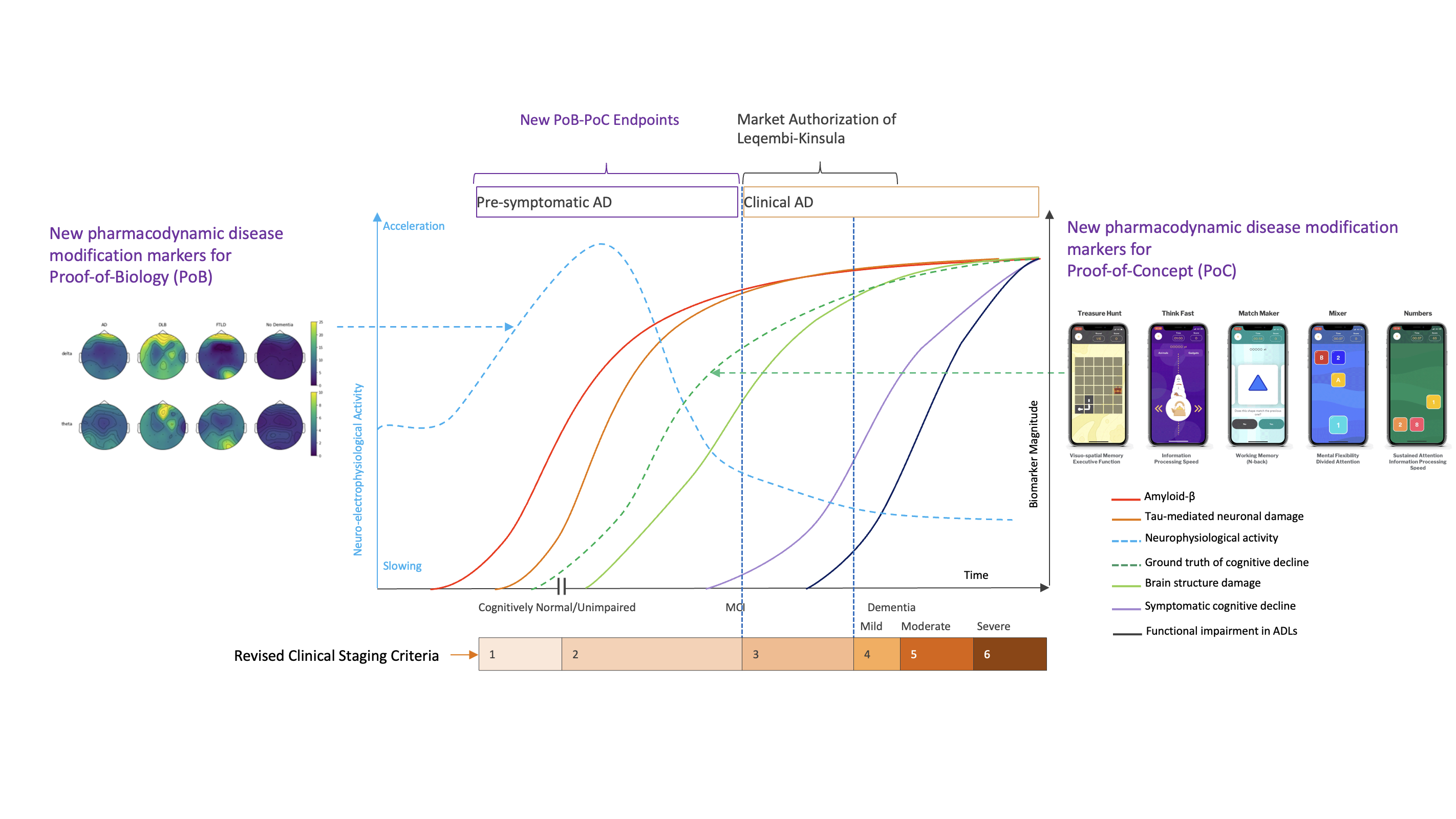

Enhancing Alzheimer’s Trials with More Responsive Pharmacodynamic Response Biomarkers of Disease Modification
Bridging Pathology and Clinical Outcomes Through Functional Brain Measurement in Early Clinical Development




Bridging Pathology and Clinical Outcomes Through Functional Brain Measurement in Early Clinical Development


Pharmaco-EEG measures the direct and indirect effects of active compounds on brain activity to evaluate a drug's pharmacodynamic (PD) and pharmacokinetic (PK) properties, its CNS penetration, and its potential therapeutic or toxic effects. By employing standardized methodologies, pharmaco-EEG can serve as a translational biomarker, bridging preclinical research and clinical drug development and aiding in the evaluation of CNS-active compounds in humans.


Electrical Source Imaging estimates the underlying brain activity from the EEG using an electromagnetic model built from the patient‘s MRI. ESI of interictal epileptiform discharges (IEDs) has been proven useful in the localization of the epileptogenic zone in the presurgical evaluation of refractory epilepsy patients, complementing magnetic resonance imaging (MRI), positron emission tomography (PET), single photon emission computed tomography (SPECT), and magnetoencephalography (MEG).


Event-related potentials (ERPs), recorded using scalp electrophysiology (EEG), offer a non-invasive, high-temporal-resolution method for assessing the cognitive and socio-emotional development in rare pediatric CNS disorders, where objective biomarkers to assess treatment response are often lacking. By capturing neural responses to sensory, cognitive, and motor stimuli, ERPs enable the quantification of dysfunctions in information processing, even in pre-verbal or minimally communicative patients. Specific ERP components, such as mismatch negativity (MMN) for auditory processing and P300 for cognitive function, can serve as biomarkers of disease progression and treatment response. Their sensitivity to subtle neural changes makes ERPs valuable endpoints for clinical trials, facilitating early detection and evaluation of therapeutic efficacy in pediatric CNS patients.


Natural History Studies (NHS) aim to enhance our understanding of specific Developmental and Epileptic Encephalopathies (DEEs). As part of an observational study, objectively phenotyping the EEG signatures of such patients can help determine disease-specific surrogate markers for disease progression and aid in developing more targeted and effective treatments.
Contact us for a talk with one of our experts.