Case studies

Enhancing Alzheimer’s Trials with More Responsive Pharmacodynamic Response Biomarkers of Disease Modification
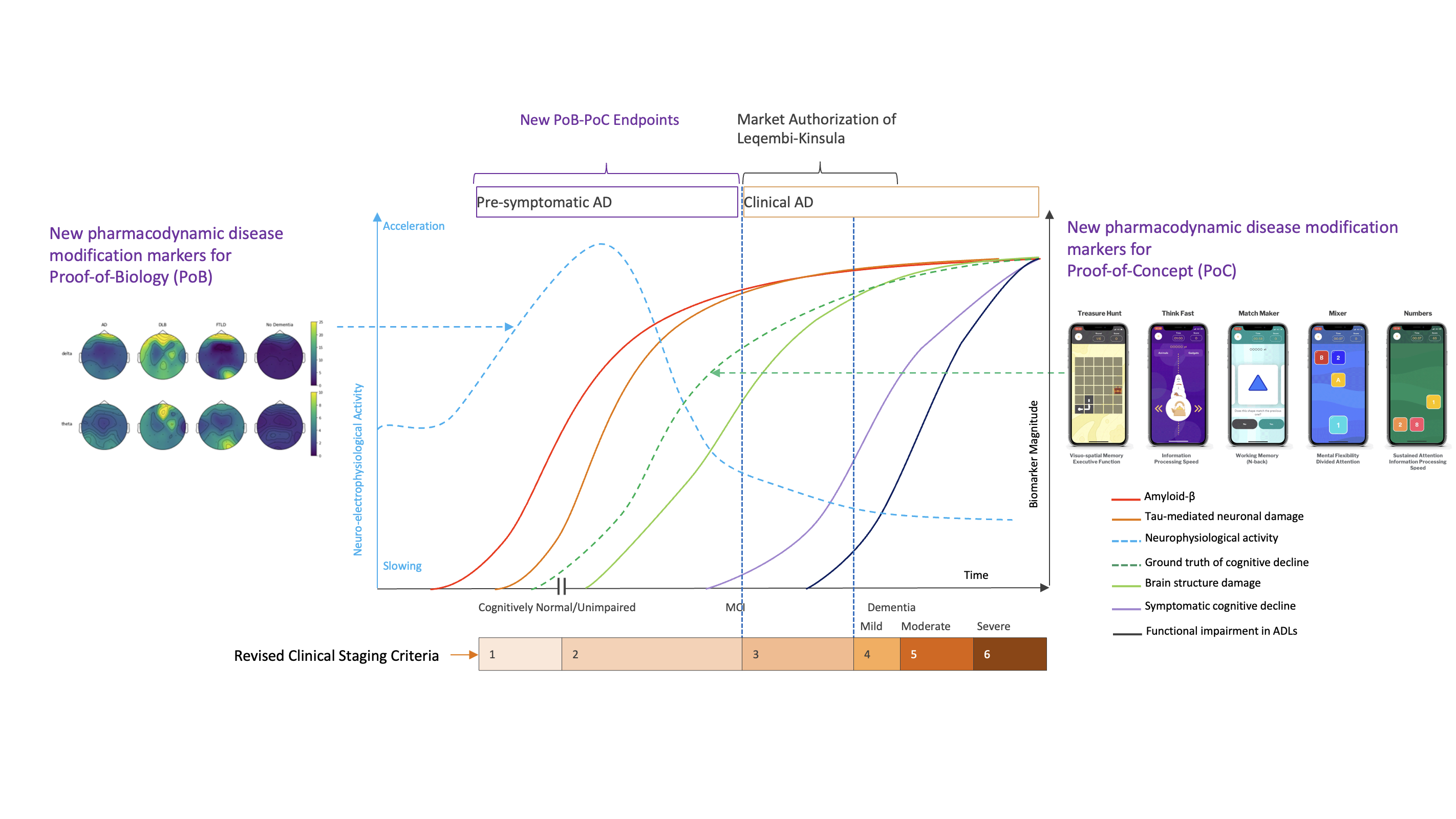
Context
Drug development in Alzheimer’s disease (AD) is currently guided by two principal categories of endpoints. On one side are biomarkers focused on the target pathobiology, such as cerebrospinal fluid (CSF) and PET imaging, with blood-based biomarkers gaining importance due to scalability and accessibility. These tools quantify underlying disease biology and are essential for demonstrating target engagement.
On the other side are clinical scales and questionnaires designed to capture cognition and daily functioning. While widely accepted, these scales are subjective and lack sensitivity to change in early-stage and slowly progressive diseases such as AD.
A translational gap persists between these two layers of evidence. Target engagement biomarkers demonstrate that a molecular process is being influenced, yet they do not directly reflect how neural circuits are functioning. Whereas clinical scales which are poorly responsive require long observation periods and large sample sizes to detect change.
Neuroelectrophysiology, captured through resting-state EEG, event-related potentials (ERP), and polysomnography (PSG), together with user-adaptive cognitive assessments, provides a functional measurement layer that bridges this gap. These modalities offer objective, real-time insight into brain activity, information processing, and sleep regulation, domains that are central to both AD pathophysiology and therapeutic response. Their role in clinical development is expanding as evidence accumulates for their sensitivity, translational relevance, and feasibility across disease stages2.
Challenge
Despite major advances in disease biology and pathology biomarkers, AD drug development continues to suffer from a very low probability of technical and regulatory success (PTRS). The highest attrition occurs in the early stages between First-in-Human, Proof of Biology, and Proof of Concept.
At this stage, many programs successfully demonstrate biological target engagement, yet fail to confirm that this engagement translates into meaningful improvement of brain activity and function. Conventional clinical endpoints lack the responsiveness required for early detection of treatment effects. As a result, early studies often operate with a low signal-to-noise ratio, making it difficult to distinguish true pharmacodynamic effects from inter-individual variability and disease progression.
This uncertainty leads to prolonged and increasingly expensive mid-stage trials that resemble registrational studies in design, scale, and costs. Development teams are frequently forced to delay key go or no-go decisions, exposing programs to significant late-stage risk and preventing timely prioritization of the most promising therapeutic candidates.
Solution
The integration of neuroelectrophysiology and adaptive cognitive assessment into early AD trials introduces a sensitive functional layer that directly captures neural system responses to therapeutic intervention.
Electrophysiological measures provide objective readouts of brain function across multiple levels, including network oscillations, synaptic integrity, sensory processing, cognitive information flow, and sleep architecture.
- Resting-state EEG enables characterization of neural oscillations.
- ERP captures stimulus-locked cognitive processing
- PSG provides insight into sleep structure and microarchitecture, which are closely linked to neurodegeneration and cognition.
Smartphone-based user-adaptive cognitive assessments complement these measures by dynamically adjusting task difficulty to individual performance, which enables to saturate learning ability and practice-related effects prior to trial baseline. This improves sensitivity to within-subject disease-related change and reduces ceiling and floor effects that commonly limit traditional cognitive assessments in early-stage AD trial populations.
When applied longitudinally, this multi-modal deep phenotyping framework enables early detection of circuit-level modulation as a marker of Proof of Biology, and functional pharmacodynamic response that support Proof of Concept of Alzheimer’s disease modification before it may become measurable on clinical scales, only later when surpassing their noise.
From an operational perspective, today’s electrophysiology setup is well-suited to AD populations. Passive paradigms are brief and well tolerated. At-home EEG, ERP, and PSG reduce site burden and improve scalability. Wearable systems facilitate overnight sleep assessment with a minimal electrode setup. Centralized acquisition, quality control, and harmonized analytics pipelines support consistency and reproducibility across sites and studies.
Impact
The incorporation of multi-modal deep phenotyping into early AD trials could deliver clear scientific and development value:
- Early functional biomarkers may strengthen the evaluation of Proof of Biology and Proof of Concept in Phase 1b and Phase 2 by increasing the sensitivity of treatment effect detection.
- Improved signal-to-noise ratio may enable clearer differentiation between dose levels and responder subgroups.
This approach holds potential to support more confident and timely development decisions with fewer patients and shorter study durations. By aligning molecular pathology, functional brain activity, and emerging cognitive change within a single translational framework, clinical development programs gain a more complete understanding of therapeutic mechanisms and impact. This integrated evidence base increases the robustness of early clinical packages and improves alignment with evolving regulatory expectations for early-stage endpoints in neurodegenerative disease.
More broadly, functional brain measures reframe early AD development from one that relies primarily on delayed clinical change to one that directly interrogates neural system modulation. This shift supports a precision-based approach to early decision-making and addresses a central driver of recurrent failure in neurodegenerative drug development.
Made possible by:


Sources:
- Figure adapted from Rafii MS et al. Alzheimer’s Dement. 2023, 19:1227–1233. Jack CR et al. Alzheimers Dement. 2024, 20:5143-5169. Dubois B et al. JAMA Neurol. 2024 Nov 1. doi: 10.1001/jamaneurol.2024.3770. Clouds of Care and Indivi data on file.
- Sohrabpour A, Sarovic D, Zetterberg H, Hämäläinen MS, Baillet S, Khan S. Tracking electrophysiological signatures of Alzheimer's disease: a systematic review of multimodal studies. Alzheimer's Dement. 2025; 21:e70835. https://doi.org/10.1002/alz.70835
A question about this case?
Contact us for a talk with one of our experts.


